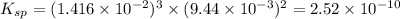Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

You know the right answer?
1.24 grams of magnesium phosphate tribasic dissolved in 1 L of lemon juice. What is the Ksp of the m...
Questions

Mathematics, 13.09.2021 02:50


English, 13.09.2021 02:50

Mathematics, 13.09.2021 02:50


Mathematics, 13.09.2021 02:50


Mathematics, 13.09.2021 02:50


Mathematics, 13.09.2021 02:50

Mathematics, 13.09.2021 02:50





Mathematics, 13.09.2021 03:00

Mathematics, 13.09.2021 03:00

English, 13.09.2021 03:00


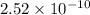
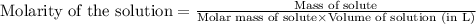
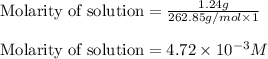
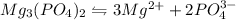
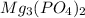 will be:
will be:![K_{sp}=[Mg^{2+}]^3[PO_4^{3-}]^2](/tpl/images/0540/2821/707f8.png)
![[Mg^{2+}]=(3\times 4.72\times 10^{-3})=1.416\times 10^{-2}M](/tpl/images/0540/2821/1537a.png)
![[PO_4^{3-}]=(2\times 4.72\times 10^{-3})=9.44\times 10^{-3}M](/tpl/images/0540/2821/66769.png)