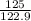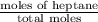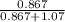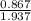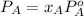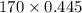Chemistry, 10.03.2020 07:25 starfox5454
At a certain temperature the vapor pressure of pure heptane (C_7 H_16) is measured to be 170. torr. Suppose a solution is prepared by mixing 86.7 g of heptane and 125. g of acetyl bromide (CH_3 COBr). Calculate the partial pressure of heptane vapor above this solution. Round your answer to 3 significant digits. Note for advanced students: you may assume the solution is ideal.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3


Chemistry, 23.06.2019 04:00
What are the names of these two interactions with cattle and how do they differ from each other
Answers: 3
You know the right answer?
At a certain temperature the vapor pressure of pure heptane (C_7 H_16) is measured to be 170. torr....
Questions


History, 18.09.2019 21:30

Mathematics, 18.09.2019 21:30


Mathematics, 18.09.2019 21:30

Mathematics, 18.09.2019 21:30


Biology, 18.09.2019 21:30

Geography, 18.09.2019 21:30



English, 18.09.2019 21:30

Mathematics, 18.09.2019 21:30

Physics, 18.09.2019 21:30

Biology, 18.09.2019 21:30



Mathematics, 18.09.2019 21:30

Biology, 18.09.2019 21:30

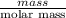
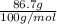
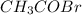 are calculated as follows.
are calculated as follows.