Chemistry, 10.03.2020 07:25 hhvgbv49551
The heat of fusion ΔHf of toluene C6H5CH3 is 6.6 /kJmol . Calculate the change in entropy ΔS when 46.g of toluene melts at −95.0°C

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3
You know the right answer?
The heat of fusion ΔHf of toluene C6H5CH3 is 6.6 /kJmol . Calculate the change in entropy ΔS when 46...
Questions



Mathematics, 10.05.2021 21:00




Social Studies, 10.05.2021 21:00

Mathematics, 10.05.2021 21:00

Mathematics, 10.05.2021 21:00

English, 10.05.2021 21:00





English, 10.05.2021 21:00

Geography, 10.05.2021 21:00

Mathematics, 10.05.2021 21:00



Mathematics, 10.05.2021 21:00

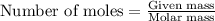
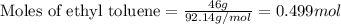
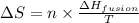
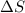 = Entropy change = ?
= Entropy change = ?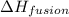 = enthalpy of fusion = 6.6 kJ/mol = 6600 J/mol (Conversion factor: 1 kJ = 1000 J)
= enthalpy of fusion = 6.6 kJ/mol = 6600 J/mol (Conversion factor: 1 kJ = 1000 J)![-95.0^oC=[-95.0+273]K=178K](/tpl/images/0540/3339/0d1c2.png)