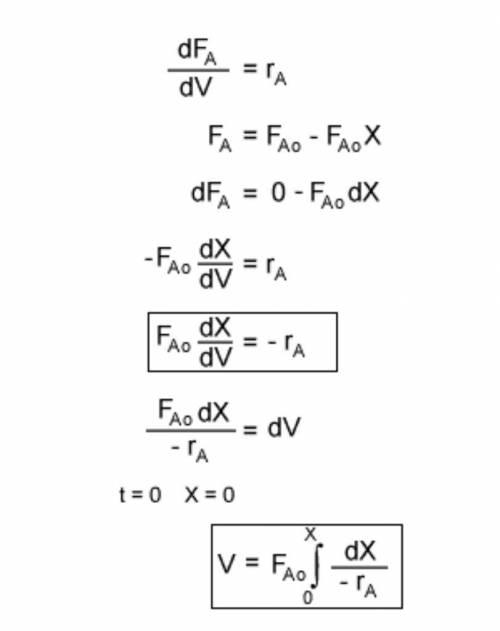
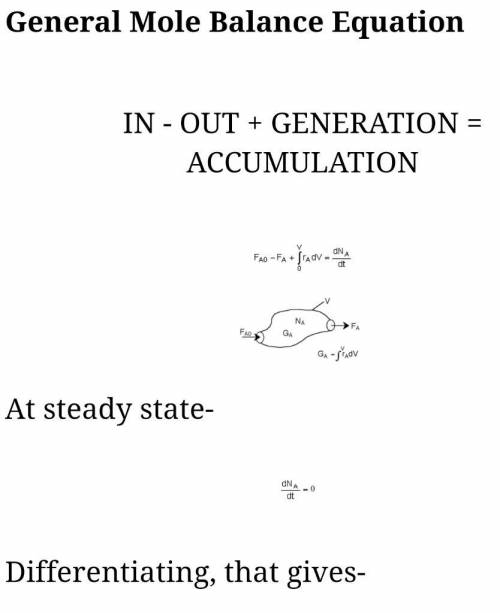
The irreversible decomposition of the di-tert-butyl peroxide is to carried out in an isothermal PFR in which there is no pressure drop. Symbolically, this reaction can be written A > B 2C. The feed consists of di-tert-butyl peroxide and inert nitrogen. The reactor volume is 200 dm3, and the entering volumetric flow rate is maintained constant at 10 dm3/min. The reaction rate constant k for this first-order reaction is 0.08 min-1 which is based on reactant A. For this reactor system, write;(a) the material balance equation(b) the rate law equation

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1
You know the right answer?
The irreversible decomposition of the di-tert-butyl peroxide is to carried out in an isothermal PFR...
Questions

Spanish, 30.11.2020 20:00



History, 30.11.2020 20:00


English, 30.11.2020 20:00

History, 30.11.2020 20:00

History, 30.11.2020 20:00


English, 30.11.2020 20:00



English, 30.11.2020 20:00

Social Studies, 30.11.2020 20:00


Biology, 30.11.2020 20:00




Mathematics, 30.11.2020 20:00