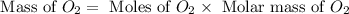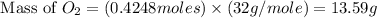Chemistry, 10.03.2020 07:34 HotWheels162000
The combustion of propane may be described by the chemical equation C 3 H 8 ( g ) + 5 O 2 ( g ) ⟶ 3 CO 2 ( g ) + 4 H 2 O ( g ) How many grams of O 2 ( g ) are needed to completely burn 89.2 g C 3 H 8 ( g ) ?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
You know the right answer?
The combustion of propane may be described by the chemical equation C 3 H 8 ( g ) + 5 O 2 ( g ) ⟶ 3...
Questions

Physics, 26.08.2021 23:50



Mathematics, 26.08.2021 23:50

Mathematics, 26.08.2021 23:50


Mathematics, 26.08.2021 23:50

Mathematics, 26.08.2021 23:50


Biology, 26.08.2021 23:50


Chemistry, 26.08.2021 23:50




Mathematics, 26.08.2021 23:50

Mathematics, 26.08.2021 23:50



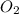 needed is, 13.59 grams.
needed is, 13.59 grams.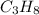 = 89.2 g
= 89.2 g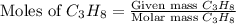
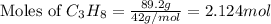
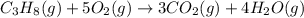
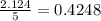 mole of
mole of