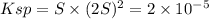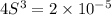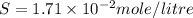Chemistry, 10.03.2020 07:33 elizediax6421
Calculate the molar solubility of lead thiocyanate in pure water. The molar solubility is the maximum amount of lead thiocyanate the solution can hold.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:30
What is the molecular formula of a hydrocarbon with m+ = 166? (write the formula with no subscripts, e.g. c4h10.) what is the sum of rings and double bonds in this compound?
Answers: 1


Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

You know the right answer?
Calculate the molar solubility of lead thiocyanate in pure water. The molar solubility is the maximu...
Questions




History, 25.12.2019 06:31


Physics, 25.12.2019 06:31




History, 25.12.2019 06:31

Mathematics, 25.12.2019 06:31


Mathematics, 25.12.2019 06:31

Health, 25.12.2019 06:31


Chemistry, 25.12.2019 06:31


Mathematics, 25.12.2019 06:31


Mathematics, 25.12.2019 06:31

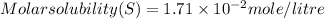
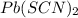 =
=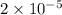 ( it is general value ,i took it because not given in question)
( it is general value ,i took it because not given in question)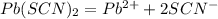
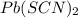 is S then
is S then![[Pb^{+2}]=S;](/tpl/images/0540/3765/fe91f.png)
![[SCN^{-1} ]=2S;](/tpl/images/0540/3765/23e37.png)