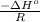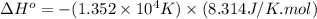Chemistry, 10.03.2020 08:19 jasonoliva13
A plot of ln(K) versus 1/T for this reaction gives a straight line with a slope of 1.352 x 104 K and a y-intercept of -14.51. Determine the value of ΔH° for this reaction.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
You know the right answer?
A plot of ln(K) versus 1/T for this reaction gives a straight line with a slope of 1.352 x 104 K and...
Questions




History, 13.05.2021 08:10

Mathematics, 13.05.2021 08:10


Mathematics, 13.05.2021 08:10


English, 13.05.2021 08:10

Mathematics, 13.05.2021 08:10

History, 13.05.2021 08:10

Mathematics, 13.05.2021 08:10





Biology, 13.05.2021 08:10


Engineering, 13.05.2021 08:10

Mathematics, 13.05.2021 08:10