
Chemistry, 10.03.2020 08:21 lovebunny33921
CO(g)+2H2(g)⇌CH3OH(g)CO(g)+2H2(g)⇌C H3OH(g) This reaction is carried out at a different temperature with initial concentrations of [CO]=0.27M[CO]=0.27M and [H2]=0.49M[H2]=0.49M. At equilibrium, the concentration of CH3OHCH3OH is 0.11 MM. Find the equilibrium constant at this temperature.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1

Chemistry, 23.06.2019 04:10
What does the field of thermodynamics relate to a-changes in nuclear reactions b- changes in energy in systems c changes in molecular structure d changes in atomic properties
Answers: 1
You know the right answer?
CO(g)+2H2(g)⇌CH3OH(g)CO(g)+2H2(g)⇌C H3OH(g) This reaction is carried out at a different temperature...
Questions

Mathematics, 20.01.2020 19:31

Spanish, 20.01.2020 19:31

Biology, 20.01.2020 19:31







Mathematics, 20.01.2020 19:31



History, 20.01.2020 19:31



Social Studies, 20.01.2020 19:31


Mathematics, 20.01.2020 19:31


History, 20.01.2020 19:31

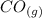 +
+ 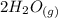 ⇄
⇄ 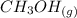
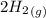 ⇄
⇄ ![K_C = \frac{[CH_3OH]}{[CO][H_2]^2}](/tpl/images/0540/6074/3667b.png)
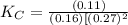
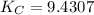
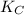 = 9.4
= 9.4

