
Chemistry, 10.03.2020 08:05 andreanaapollon6337
A gaseous compound containing carbon and hydrogen was analyzed and found to consist of 83.65% carbon by mass. What is the empirical formula of the compound

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 23.06.2019 03:30
In general metals get as you move from left to right across the periodic table.
Answers: 1
You know the right answer?
A gaseous compound containing carbon and hydrogen was analyzed and found to consist of 83.65% carbon...
Questions

Health, 21.10.2020 14:01

Mathematics, 21.10.2020 14:01

Mathematics, 21.10.2020 14:01

Computers and Technology, 21.10.2020 14:01

Mathematics, 21.10.2020 14:01







Biology, 21.10.2020 14:01

Arts, 21.10.2020 14:01

Mathematics, 21.10.2020 14:01

English, 21.10.2020 14:01

Mathematics, 21.10.2020 14:01

Biology, 21.10.2020 14:01


English, 21.10.2020 14:01

Business, 21.10.2020 14:01

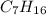
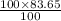 is 83.65 gram
.
is 83.65 gram
.

