
Chemistry, 10.03.2020 08:25 darius12318
Arrange the molecules H2O, NH3, Ar, NaCl in order of expected increasing boiling points. 1. None of these 2. NaCl, H2O, NH3, Ar 3. Ar, NH3, H2O, NaCl 4. NH3, Ar, H2O, NaCl

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3


You know the right answer?
Arrange the molecules H2O, NH3, Ar, NaCl in order of expected increasing boiling points. 1. None of...
Questions

Chemistry, 21.09.2019 01:50




Spanish, 21.09.2019 01:50


History, 21.09.2019 01:50


Business, 21.09.2019 01:50

English, 21.09.2019 01:50





Mathematics, 21.09.2019 02:00

Biology, 21.09.2019 02:00

Mathematics, 21.09.2019 02:00

Mathematics, 21.09.2019 02:00


 and
and 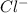 ions are held together by strong coloumbic attractive force. Hence NaCl has highest boiling point'Ar is a monoatomic molecule. Hence only weak london dispersion force exists between Ar atom/molecules. Hence it requires lowest amount of energy to boil and thereby possess lowest boiling point.
ions are held together by strong coloumbic attractive force. Hence NaCl has highest boiling point'Ar is a monoatomic molecule. Hence only weak london dispersion force exists between Ar atom/molecules. Hence it requires lowest amount of energy to boil and thereby possess lowest boiling point. and
and  are polar protic molecules. Hence they possess london dispersion force, dipole-dipole force and hydrogen bonding as intermolecular forces.Dipole-dipole force is stronger in
are polar protic molecules. Hence they possess london dispersion force, dipole-dipole force and hydrogen bonding as intermolecular forces.Dipole-dipole force is stronger in 


