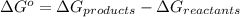For the following reaction, label each of the below species as an acid or a base. Use lower case letters only (e. g. acid)
HCN + HPO4⁻² ⇔ H2PO4⁻ + CN⁻
Will the above reaction take place spontaneously? (Is the reaction product-favored? Does the equilibrium lie to the right?)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2
You know the right answer?
For the following reaction, label each of the below species as an acid or a base. Use lower case let...
Questions


Mathematics, 28.05.2020 19:58


Mathematics, 28.05.2020 19:58

Health, 28.05.2020 19:58


Mathematics, 28.05.2020 19:58





World Languages, 28.05.2020 19:58

Biology, 28.05.2020 19:58



Geography, 28.05.2020 19:58



Chemistry, 28.05.2020 19:58

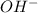 .
. 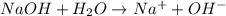
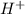 .
.
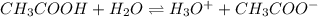
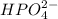 is accepting the hydrogen ions so it acts as a base.
is accepting the hydrogen ions so it acts as a base.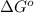 values of the given species are as follows.
values of the given species are as follows.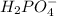 = -1130.4 kJ/mol,
= -1130.4 kJ/mol, 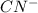 = 172.4 kJ/mol
= 172.4 kJ/mol