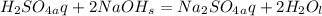Problem PageQuestion Aqueous sulfuric acid will react with solid sodium hydroxide to produce aqueous sodium sulfate and liquid water . Suppose 91. g of sulfuric acid is mixed with 116. g of sodium hydroxide. Calculate the maximum mass of sodium sulfate that could be produced by the chemical reaction. Round your answer to significant digits.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3
You know the right answer?
Problem PageQuestion Aqueous sulfuric acid will react with solid sodium hydroxide to produce aqueous...
Questions

Mathematics, 05.07.2019 09:10




English, 05.07.2019 09:10

Mathematics, 05.07.2019 09:10

Mathematics, 05.07.2019 09:10

English, 05.07.2019 09:10


Mathematics, 05.07.2019 09:10


Mathematics, 05.07.2019 09:10

Health, 05.07.2019 09:10


History, 05.07.2019 09:10

Social Studies, 05.07.2019 09:10

World Languages, 05.07.2019 09:10

Mathematics, 05.07.2019 09:10

Mathematics, 05.07.2019 09:10