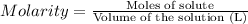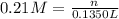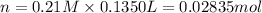Chemistry, 10.03.2020 08:58 jdodger5165
A chemist adds 135.0 mL of a 0.21M zinc nitrate (Zn(NO3) solution to a reaction flask. Calculate the mass in grams of zinc nitrate the chemist has added to the flask. Be sure your answer has the correct number of significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 18:30
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1
You know the right answer?
A chemist adds 135.0 mL of a 0.21M zinc nitrate (Zn(NO3) solution to a reaction flask. Calculate the...
Questions


Mathematics, 29.09.2020 01:01

Mathematics, 29.09.2020 01:01

English, 29.09.2020 01:01

History, 29.09.2020 01:01

Engineering, 29.09.2020 01:01



Mathematics, 29.09.2020 01:01




Biology, 29.09.2020 01:01

History, 29.09.2020 01:01



Mathematics, 29.09.2020 01:01

Health, 29.09.2020 01:01