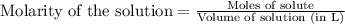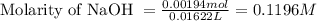A 0.397-g sample of potassium hydrogen phthalate, KHC8H4O4 (molar mass = 204.22 g/mol) is dissolved with 50 mL of deionized water in a 125-mL Erlenmeyer flask. The sample is titrated to the phenolphthalein endpoint with 16.22 mL of a sodium hydroxide solution. What is the molar concentration of the NaOH solution? (Show all calculations)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3
You know the right answer?
A 0.397-g sample of potassium hydrogen phthalate, KHC8H4O4 (molar mass = 204.22 g/mol) is dissolved...
Questions


History, 25.01.2021 01:00

Mathematics, 25.01.2021 01:00

English, 25.01.2021 01:00


Mathematics, 25.01.2021 01:00


Physics, 25.01.2021 01:00

Mathematics, 25.01.2021 01:00


Chemistry, 25.01.2021 01:00

English, 25.01.2021 01:00


Mathematics, 25.01.2021 01:00

Mathematics, 25.01.2021 01:00

Mathematics, 25.01.2021 01:00


Physics, 25.01.2021 01:00


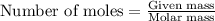
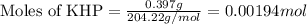
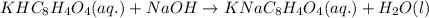
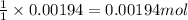 of NaOH.
of NaOH.