
50.0 mL solution of 0.160 M potassium alaninate ( H 2 NC 2 H 5 CO 2 K ) is titrated with 0.160 M HCl . The p K a values for the amino acid alanine are 2.344 ( p K a1 ) and 9.868 ( p K a2 ) , which correspond to the carboxylic acid and amino groups, respectively. a) how do you calculate the PH of the first equivalent and b) the second equivalent? please help

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2


Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2
You know the right answer?
50.0 mL solution of 0.160 M potassium alaninate ( H 2 NC 2 H 5 CO 2 K ) is titrated with 0.160 M HCl...
Questions


Mathematics, 04.05.2021 03:30




Mathematics, 04.05.2021 03:30


English, 04.05.2021 03:30



Arts, 04.05.2021 03:30

Mathematics, 04.05.2021 03:30

Mathematics, 04.05.2021 03:30


Spanish, 04.05.2021 03:30


Mathematics, 04.05.2021 03:30


Mathematics, 04.05.2021 03:30

Computers and Technology, 04.05.2021 03:30

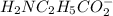

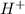 ------>
------> 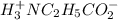
![[H_3}^+NC_2H_5CO^-_2]](/tpl/images/0540/7336/f75f8.png) =
= 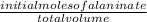
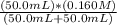
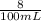
![[H^+]](/tpl/images/0540/7336/07acb.png) =
= ![\sqrt{\frac{K_{a1}K_{a2}{[H_3}^+NC_2H_5CO^-_2]+K_{a1}K_w}{ K_{a1}{[H_3}^+NC_2H_5CO^-_2] } }](/tpl/images/0540/7336/a0e17.png)
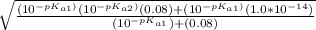

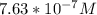
![-log[7.63*10^{-7}]](/tpl/images/0540/7336/a344f.png)
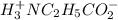
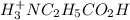
![[H^+_3NC_2H_5CO_2H]](/tpl/images/0540/7336/4ff0a.png) =
= 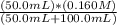
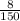
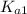 =
= ![\frac{[H^+] [H^+_3NC_2H_5CO^-_2]}{[H^+_3NC_2H_5CO_2H]}](/tpl/images/0540/7336/e2bce.png)
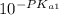 =
= 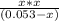
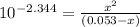
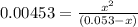
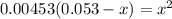
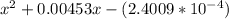
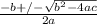
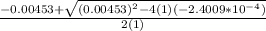 OR
OR 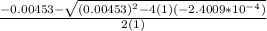
![[H^+]=[H_3^+NC_2H_5CO^-_2]= 0.0134 M](/tpl/images/0540/7336/73fad.png)
![-log[H^+]](/tpl/images/0540/7336/cbdd4.png)
![-log[0.0134]](/tpl/images/0540/7336/6b2f8.png)


