Chemistry, 10.03.2020 09:10 nails4life324
Which of the following equilibrium expressions corresponds to a heterogeneous equilibrium?(a) Kc =[NH4+][OH−][NH3](b) Kc =[H+][C2H3O2−][HC2H3O2](c) Kc =[Ag(NH3)2+][Cl−][NH3]2(d) Kc = [Ba2+][F−]2

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
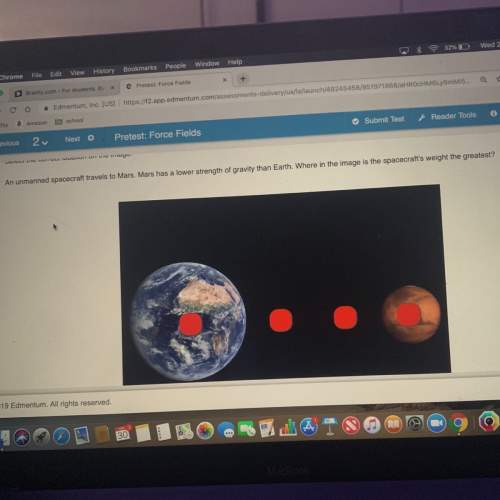
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

You know the right answer?
Which of the following equilibrium expressions corresponds to a heterogeneous equilibrium?(a) Kc =[N...
Questions

Mathematics, 23.06.2021 21:00



English, 23.06.2021 21:00

Mathematics, 23.06.2021 21:00

Mathematics, 23.06.2021 21:00

Mathematics, 23.06.2021 21:00




History, 23.06.2021 21:00




Biology, 23.06.2021 21:00





English, 23.06.2021 21:00

![Kc = \frac{[Ag(NH_{3})_2^{+}][Cl^-]}{[NH_3]^2}}](/tpl/images/0540/7502/cbebb.png)
![Kc = [Ba^{2+}][F^{-}]^2](/tpl/images/0540/7502/a3116.png)
![Kc = \frac{[NH_{4}^+][OH^-]}{[NH_3]}}](/tpl/images/0540/7502/3684d.png)
![Kc = \frac{[H^+][C_2H_3O_{2}^-]}{[HC_2H_3O_2]}}](/tpl/images/0540/7502/8b1f8.png)