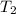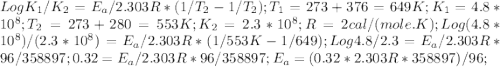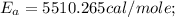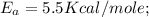Chemistry, 10.03.2020 18:49 kkwolfcityouwc96
The rate constant k for a certain reaction is measured at two different temperatures:
temperature k
376.0 °c 4.8 x 108
280.0 °C 2.3 x 10 8
Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy Ea for this reaction.
Round your answer to 2 significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3
You know the right answer?
The rate constant k for a certain reaction is measured at two different temperatures:
te...
te...
Questions






Biology, 20.10.2021 14:00


Mathematics, 20.10.2021 14:00

Mathematics, 20.10.2021 14:00

Mathematics, 20.10.2021 14:00




Biology, 20.10.2021 14:00



Arts, 20.10.2021 14:00


Mathematics, 20.10.2021 14:00

Computers and Technology, 20.10.2021 14:00

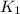 and
and 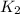 at temperature
at temperature 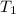 and
and