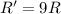Chemistry, 10.03.2020 19:06 ryliekarenbowers
By what factor does the rate change in each of the following cases (assuming constant temperature)?
Factor (enter as decimal if <1)
(a) A reaction is first order in reactant A, and [A] is doubled.
(b) A reaction is second order in reactant B, and [B] is halved.
(c) A reaction is second order in reactant C, and [C] is tripled.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1


Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
You know the right answer?
By what factor does the rate change in each of the following cases (assuming constant temperature)?...
Questions






History, 25.11.2021 05:10

Biology, 25.11.2021 05:10


Computers and Technology, 25.11.2021 05:10

Physics, 25.11.2021 05:10

Computers and Technology, 25.11.2021 05:10


Physics, 25.11.2021 05:10

Physics, 25.11.2021 05:10

Mathematics, 25.11.2021 05:10



Mathematics, 25.11.2021 05:10


![R=k[A]^1](/tpl/images/0541/2780/9975f.png)
![R'=k[2A]^1](/tpl/images/0541/2780/53afe.png)
![R'=2k[A]](/tpl/images/0541/2780/37f63.png)
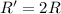
![R=k[B]^2](/tpl/images/0541/2780/92409.png)
![R'=k[\frac{B}{2}]^2](/tpl/images/0541/2780/d1975.png)
![R'=\frac{1}{4}k[B]^2](/tpl/images/0541/2780/1571d.png)
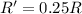
![R=k[C]^2](/tpl/images/0541/2780/01b2f.png)
![R'=k[3C]^2](/tpl/images/0541/2780/92d86.png)
![R'=9k[C]^2](/tpl/images/0541/2780/6f960.png)