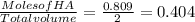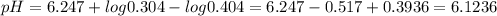Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1


Chemistry, 23.06.2019 12:30
Growing crops in places where major pests don't live using beneficial insects to eat harmful insects using a rat trap instead of a rodenticide developing drought-resistant tomato plants using beneficial insects or natural oils to repel pests planting a different crop every year to fake out the pests keeping food covered to deter ants and rodents developing bean plants with a resistance to fungus picking caterpillars off tomato plants cultivation practice biological control cultural control genetic resistance natural chemicals
Answers: 3
You know the right answer?
What is the ph of a buffer prepared by adding 0.809 mol of the weak acid ha to 0.608 mol of naa in 2...
Questions

History, 02.06.2020 02:57


Social Studies, 02.06.2020 02:57



Mathematics, 02.06.2020 02:57



Mathematics, 02.06.2020 02:57

Mathematics, 02.06.2020 02:57

Mathematics, 02.06.2020 02:57

History, 02.06.2020 02:57


Social Studies, 02.06.2020 02:57


Mathematics, 02.06.2020 02:57

Mathematics, 02.06.2020 02:57

English, 02.06.2020 02:57


![pKa=-log[H] = - log [ 5.66 * 10^{-7}]\\ \\pka = 7 - log (5.66)=7-0.753=6.247\\\\pka = 6.247](/tpl/images/0541/2737/077ac.png)
![pH = pK_{a} + log[\frac{[A-]}{[HA]}}]](/tpl/images/0541/2737/65a3d.png)
![[A^{-}] = Moles of [A]/Total volume = 0.608/2 = 0.304 M\\](/tpl/images/0541/2737/854a1.png)