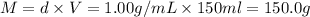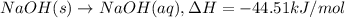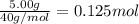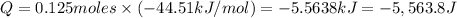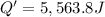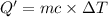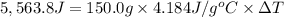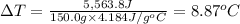Chemistry, 10.03.2020 19:01 gooberthebear8955
The enthalpy of solution (dissolving) of sodium hydroxide is given below. Determine the change in temperature of a coffee cup calorimeter containing 150 ml of water when 5.00 g NaOH (40.00 g/mol) is added to the container. You may assume that the solution has the same specific heat and density as water.
NaOH(s) → NaOH(aq) ΔH =-44.51 KJ
a. +8.87°C
b. +2.70 C
c. 2.70°C
d. 8.87°C

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
You know the right answer?
The enthalpy of solution (dissolving) of sodium hydroxide is given below. Determine the change in te...
Questions

Computers and Technology, 16.04.2020 12:20


English, 16.04.2020 12:39

History, 16.04.2020 12:40

Chemistry, 16.04.2020 12:40

History, 16.04.2020 12:41



Mathematics, 16.04.2020 12:41


Mathematics, 16.04.2020 12:41

English, 16.04.2020 12:42

Mathematics, 16.04.2020 12:42




Biology, 16.04.2020 12:42



Mathematics, 16.04.2020 12:42