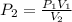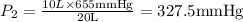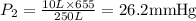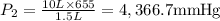Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2


Chemistry, 23.06.2019 14:00
Cassandra made a venn diagram to compare and contrast the two stages of cellular respiration. which belongs in the area marked x? energy is released. oxygen is used up. glucose is broken down. carbon dioxide is used up.
Answers: 1

Chemistry, 23.06.2019 14:20
Timed ! in which of these statements are protons, electrons, and neutrons correctly compared? quarks are present in protons and neutrons but not in electrons. quarks are present in protons, neutrons, and electrons. quarks are present in neutrons and electrons but not in protons. quarks are present in protons and electrons but not in neutrons.
Answers: 1
You know the right answer?
A 10.0-L balloon contains helium gas at a pressure of 655 mmHg. What is the new pressure, in mmHg, o...
Questions













History, 17.07.2020 20:01

Mathematics, 17.07.2020 20:01


Mathematics, 17.07.2020 20:01

Advanced Placement (AP), 17.07.2020 20:01



 10 = P2
10 = P2