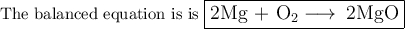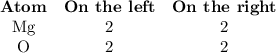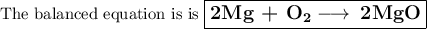Pure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. When 3.44 g of magnesium ribbon burns with 6.82 gof oxygen, a bright, white light and a white, powdery product are formed. Enter the balanced chemical equation for this reaction. Be sure to include all physical states.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
You know the right answer?
Pure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. When 3...
Questions







Health, 25.05.2021 14:00

History, 25.05.2021 14:00


Chemistry, 25.05.2021 14:00

Mathematics, 25.05.2021 14:00



Mathematics, 25.05.2021 14:00


Computers and Technology, 25.05.2021 14:00



Mathematics, 25.05.2021 14:00

Biology, 25.05.2021 14:00