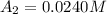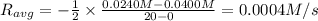Chemistry, 10.03.2020 22:12 chloesmolinski0909
(a) The rate of the reaction in terms of the "disappearance of reactant" includes the change in the concentration of the
reactant, the time interval, and the coefficient of the reactant.
Consider the following reaction:
2A+3B > 3C+2D
The concentrations of reactant A at three different time intervals are given. Use the following data to determine the average rate of reaction in terms of the disappearance of reactant A between time = 0 s and time = 20 s .
Time (s) 0 20 40
[A](M) 0.0400 0.0240 0.0180
Express your answer in molar concentration per second to three significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

You know the right answer?
(a) The rate of the reaction in terms of the "disappearance of reactant" includes the change in the...
Questions


Social Studies, 18.04.2020 20:42





Mathematics, 18.04.2020 20:43

Chemistry, 18.04.2020 20:43

Mathematics, 18.04.2020 20:43



English, 18.04.2020 20:43

Mathematics, 18.04.2020 20:43

Physics, 18.04.2020 20:43

History, 18.04.2020 20:43





Biology, 18.04.2020 20:43

![R_{avg}=-\frac{[A]_2-[A]_1}{t_2-t_1}](/tpl/images/0541/5709/9f5c2.png)
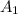 = initial concentration of reactant at
= initial concentration of reactant at 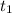 .
.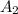 = Final concentration of reactant at
= Final concentration of reactant at 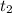 .
.![R_{avg}=-\frac{1}{2}\frac{[A]_2-[A]_1}{t_2-t_1}](/tpl/images/0541/5709/30159.png)
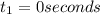 ) =
) = 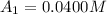
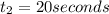 ) =
) =