
Chemistry, 10.03.2020 22:15 taniyahbenyamin2
Data for the decomposition of hydrogen peroxide at some set temperature T is provided below. The rate law depends only on the concentration of H2O2. (These same data will be used for questions 6, 7 and 8.) 2 H2O2 ---> 2 H2O O2 t (seconds) 0 60 120 180 240 360 420 600 [H2O2] (M) 0.882 0.697 0.566 0.458 0.372 0.236 0.188 0.094 What is the value of the rate constant, k

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2
You know the right answer?
Data for the decomposition of hydrogen peroxide at some set temperature T is provided below. The rat...
Questions






Mathematics, 18.01.2020 06:31

Social Studies, 18.01.2020 06:31



Chemistry, 18.01.2020 06:31

Biology, 18.01.2020 06:31

Computers and Technology, 18.01.2020 06:31

History, 18.01.2020 06:31


Mathematics, 18.01.2020 06:31

History, 18.01.2020 06:31


Mathematics, 18.01.2020 06:31


Social Studies, 18.01.2020 06:31

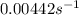 is the value of the rate constant.
is the value of the rate constant.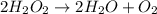
![R=k[H_2O_2]^x](/tpl/images/0541/5739/94d73.png)
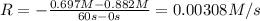
![0.00308 M/s=k[0.697 M]^x](/tpl/images/0541/5739/443b7.png) ..[1]
..[1]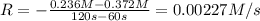
![0.00218 M/s=k[0.236 M]^x](/tpl/images/0541/5739/bccb9.png) ..[2]
..[2]![\frac{0.00308 M/s}{0.00227 M/s}=\frac{k[0.697 M]^x}{k[0.236M]^x}](/tpl/images/0541/5739/1e2cd.png)
![R=k[H_2O_2]^1](/tpl/images/0541/5739/48c78.png)
![0.00308 M/s=k[0.697 M]^1](/tpl/images/0541/5739/15c46.png)
![k=\frac{0.00308 M/s}{[0.697 M]^1}=0.00442 s^{-1}](/tpl/images/0541/5739/22700.png)


