The dissolution of PbI2(s) is represented above.

Chemistry, 10.03.2020 22:29 WhiteWinterRose
PbI2(s)⇄Pb2+(aq)+2I−(aq) Ksp=7×10−9
The dissolution of PbI2(s) is represented above.
(a) Write a mathematical expression that can be used to determine the value of S , the molar solubility of PbI2(s) .
(b) If PbI2(s) is dissolved in 1.0MNaI(aq), is the maximum possible concentration of Pb2+(aq) in the solution greater than, less than, or equal to the concentration of Pb2+(aq) in the solution in part (a) ? Explain.
Compound Ksp
PbCl2 2×10−5
PbI2 7×10−9
Pb(IO3)2 3×10−13
c) A table showing Ksp values for several lead compounds is given above. A saturated solution of which of the compounds has the greatest molar concentration of Pb2+(aq) ? Explain.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
PbI2(s)⇄Pb2+(aq)+2I−(aq) Ksp=7×10−9
The dissolution of PbI2(s) is represented above.
The dissolution of PbI2(s) is represented above.
Questions


Mathematics, 15.10.2019 08:50


English, 15.10.2019 08:50

Spanish, 15.10.2019 08:50

Mathematics, 15.10.2019 08:50


History, 15.10.2019 08:50

History, 15.10.2019 08:50



Mathematics, 15.10.2019 08:50

Social Studies, 15.10.2019 08:50





Chemistry, 15.10.2019 08:50

English, 15.10.2019 08:50

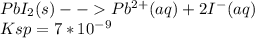
![K_s_p = [PB][I]^{2+}\\= s*2s\\s^3 = \frac{k_s_p}{4}](/tpl/images/0541/5945/0b3d5.png)
![s= \sqrt[3]{\frac{k_s_p}{4}}](/tpl/images/0541/5945/16063.png)
![s= \sqrt[3]{\frac{k_s_p}{4}}\\\\s= \sqrt[3]{\frac{7*10^-^9}{4}}](/tpl/images/0541/5945/27998.png)
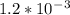 the maximum possible concentration of Pb²⁺ in the solution is always less than that in the solution in part (a). That is because of the common ion effect. The added iodide ion forces the position of equilibrium to shift to the left, reducing the concentration of Pb²⁺.
the maximum possible concentration of Pb²⁺ in the solution is always less than that in the solution in part (a). That is because of the common ion effect. The added iodide ion forces the position of equilibrium to shift to the left, reducing the concentration of Pb²⁺.![K_s_p = [Pb{^2^+}][X^-]^2](/tpl/images/0541/5945/b46ba.png)
![s =\sqrt [3]{\dfrac{K_{sp}}{4}}](/tpl/images/0541/5945/67f28.png)
![K_{sp} =\text{[Pb$^{2+}$][I$^{-}$]}^{2} = s\times (2s)^{2} = 4s^{3}\\s^{3} = \dfrac{K_{sp}}{4}\\\\s =\mathbf{ \sqrt [3]{\dfrac{K_{sp}}{4}}}\\\\\text{A mathematical expression you could use is }\mathbf{s =\sqrt [3]{\dfrac{K_{sp}}{4}}}](/tpl/images/0541/5945/67153.png)
![\begin{array}{rcl}\\s &=&\sqrt [3]{\dfrac{K_{\text{sp}}}{4}}\\\\s &=&\sqrt [3]{\dfrac{7 \times 10^{-9}}{4}}\\\\s &=&\sqrt [3]{1.75 \times 10^{-9}}\\\\&=& \mathbf{1.21 \times 10^{-3}} \textbf{ mol/L}\\\end{array}](/tpl/images/0541/5945/cc365.png)
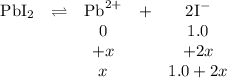
![K_{\text{sp}} =\text{[Pb$^{2+}$][I$^{-}$]}^{2} = s\times (1.0 + 2s)^{2} = 7 \times 10^{-9}\\s = \mathbf{7 \times 10^{-9}} \textbf{ mol/L}](/tpl/images/0541/5945/ddbab.png)
![K_{\text{sp}} =\text{[Pb$^{2+}$][X$^{-}$]}^{2}\\\\s = \text{[Pb$^{2+}$]} = \sqrt [3]{\dfrac{K_{\text{sp}}}{4}}](/tpl/images/0541/5945/770fe.png)


