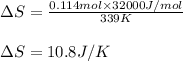Chemistry, 10.03.2020 23:02 Reebear8372
The heat of vaporization ΔΗν of tetrahydrofuran (C4H80) is 32.0 kJ/mol. Calculate the change in entropy AS when 8.2 g of tetrahydrofuran boils at 66.0 °C Be sure your answer contains a unit symbol and the correct number of significant digits.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
You know the right answer?
The heat of vaporization ΔΗν of tetrahydrofuran (C4H80) is 32.0 kJ/mol. Calculate the change in entr...
Questions

History, 06.12.2019 02:31

Biology, 06.12.2019 02:31

History, 06.12.2019 02:31

Biology, 06.12.2019 02:31




Social Studies, 06.12.2019 02:31



French, 06.12.2019 02:31



Mathematics, 06.12.2019 02:31

Mathematics, 06.12.2019 02:31




Mathematics, 06.12.2019 02:31

Business, 06.12.2019 02:31

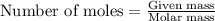
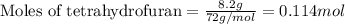
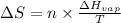
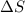 = Entropy change = ?
= Entropy change = ?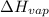 = enthalpy of vaporization = 32.0 kJ/mol = 32000 J/mol (Conversion factor: 1 kJ = 1000 J)
= enthalpy of vaporization = 32.0 kJ/mol = 32000 J/mol (Conversion factor: 1 kJ = 1000 J)![66.0^oC=[66+273]K=339K](/tpl/images/0541/6883/b2b6e.png)