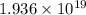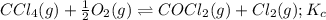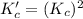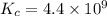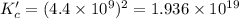Chemistry, 11.03.2020 01:06 alexandria3498
Carbon tetrachloride reacts at high temperatures with oxygen to produce two toxic gases, phosgene and chlorine. CCl4(g) + (1/2)O2(g) <> COCl2(g) + Cl2(g), with Kc = 4.4 x 109 at 1,000 K

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1
You know the right answer?
Carbon tetrachloride reacts at high temperatures with oxygen to produce two toxic gases, phosgene an...
Questions

Mathematics, 20.03.2021 07:00

Physics, 20.03.2021 07:00

English, 20.03.2021 07:00




History, 20.03.2021 07:00

Arts, 20.03.2021 07:00

Mathematics, 20.03.2021 07:10

English, 20.03.2021 07:10




English, 20.03.2021 07:10


Chemistry, 20.03.2021 07:10

English, 20.03.2021 07:10


Mathematics, 20.03.2021 07:10

English, 20.03.2021 07:10

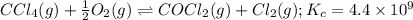 at 1,000 K
at 1,000 K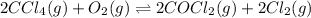
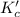 for the final reaction is
for the final reaction is