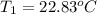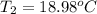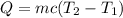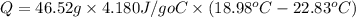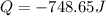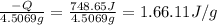Chemistry, 11.03.2020 02:11 zacharycheyne
The heat of solution is found by adding a salt to water in a calorimeter and measuring the temperature change. The specific heat of water is 4.180 Joules per g per ºC. In the calculation of the heat of solution, ignore the contribution to specific heat and mass due to the salt. Assume that these contributions are negligible. The data collected are as follows:
Grams of water in the calorimeter 46.52
Grams of salt 4.5069
Initial temperature of water 22.83 ºC
Final Temperature 18.98 ºC
Calculate the following Heat of the solution of salt.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 23.06.2019 03:30
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1

Chemistry, 23.06.2019 05:00
Which of the following describes qualitative data? a) recording the temperature of a solid as it is warmed. b) noting the color of a solution as it is heated. c) measuring the volume of an object by water displacement. d) taking the mass of an object using a balance.
Answers: 2
You know the right answer?
The heat of solution is found by adding a salt to water in a calorimeter and measuring the temperatu...
Questions

Mathematics, 21.08.2019 02:30

History, 21.08.2019 02:30

Mathematics, 21.08.2019 02:30

English, 21.08.2019 02:30




Mathematics, 21.08.2019 02:30

History, 21.08.2019 02:30


Social Studies, 21.08.2019 02:30

English, 21.08.2019 02:30

Mathematics, 21.08.2019 02:30

History, 21.08.2019 02:30




History, 21.08.2019 02:30