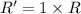In acidic solution, the breakdown of sucrose into glucose and fructose has this rate law: rate = k[H+][sucrose].
The initial rate of sucrose breakdown is measured in a solution that is 0.01 M H+, 1.0 M sucrose, 0.1 M fructose, and 0.1 M glucose.
How does the rate change if:
(a) [Sucrose] is changed to 2.5 M?
(b) [Sucrose], [fructose], and [glucose] are all changed to 0.5 M?
(c) [H+] is changed to 0.0001 M?
(d) [Sucrose] and [H+] are both changed to 0.1 M ?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2

Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
In acidic solution, the breakdown of sucrose into glucose and fructose has this rate law: rate = k[H...
Questions





English, 14.04.2020 19:24




Mathematics, 14.04.2020 19:24





Mathematics, 14.04.2020 19:24

Mathematics, 14.04.2020 19:24


Mathematics, 14.04.2020 19:24



![[H^+]](/tpl/images/0542/2176/07acb.png) is changed to 0.0001 M than rate will be increased by the factor of 0.01.
is changed to 0.0001 M than rate will be increased by the factor of 0.01.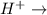 fructose+ glucose
fructose+ glucose![R=k[H^+][sucrose]](/tpl/images/0542/2176/8ca17.png)
![[H^+]=0.01M](/tpl/images/0542/2176/8ae83.png)
![R=k[0.01M][1.0 M]](/tpl/images/0542/2176/7a749.png) ..[1]
..[1]![R'=[0.01 M][2.5 M]](/tpl/images/0542/2176/5a418.png) ..[2]
..[2]![\frac{R'}{R}=\frac{[0.01 M][2.5 M]}{k[0.01M][1.0 M]}](/tpl/images/0542/2176/ac5ef.png)
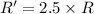
![R'=[0.01 M][0.5 M]](/tpl/images/0542/2176/d8698.png) ..[2]
..[2]![\frac{R'}{R}=\frac{[0.01 M][0.5 M]}{k[0.01M][1.0 M]}](/tpl/images/0542/2176/036aa.png)
![R'=[0.0001 M][1.0 M]](/tpl/images/0542/2176/62405.png) ..[2]
..[2]![\frac{R'}{R}=\frac{[0.0001 M][1.0M]}{k[0.01M][1.0 M]}](/tpl/images/0542/2176/7dc89.png)
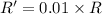
![R'=[0.1M][0.1M]](/tpl/images/0542/2176/67bb4.png) ..[2]
..[2]![\frac{R'}{R}=\frac{[0.1M][0.1M]}{k[0.01M][1.0 M]}](/tpl/images/0542/2176/6860b.png)