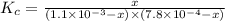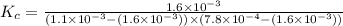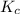Consider the following reaction:
Fe3+(aq)+SCN−(aq) <> FeSCN2+(aq)
A solution is ma...

Chemistry, 11.03.2020 02:42 Chewbacka2020
Consider the following reaction:
Fe3+(aq)+SCN−(aq) <> FeSCN2+(aq)
A solution is made containing an initial [Fe3+] of 1.1 x 10^−3 M and an initial [SCN−] of 7.8 x 10^−4 M . At equilibrium, [FeSCN2+]= 1.6 x 10^−4 M .
Part A) Calculate the value of the equilibrium constant (Kc).
Express your answer using two significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3
You know the right answer?
Questions

Chemistry, 31.08.2020 02:01








Computers and Technology, 31.08.2020 02:01


Mathematics, 31.08.2020 02:01

Mathematics, 31.08.2020 02:01







History, 31.08.2020 02:01

 .
.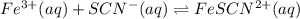
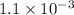
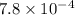 0
0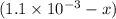
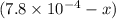 x
x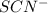 at equilibrium is given ,x=
at equilibrium is given ,x=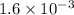
![K_c=\frac{[FeSCN^{2+}]}{[Fe^{3+}][SCN^-]}](/tpl/images/0542/1843/417f5.png)