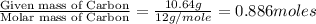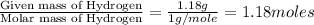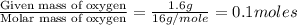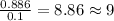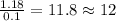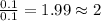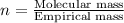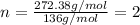Chemistry, 11.03.2020 03:31 choudharykaran7997
Combustion analysis of a 13.42-g sample of the unknown organic compound (which contains only carbon, hydrogen, and oxygen) produced 39.01 g CO2 and
10.65 g H2O. The molar mass of the unknown compound is 272.38 g/mol.
Find the molecular formula of the unknown compound.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

You know the right answer?
Combustion analysis of a 13.42-g sample of the unknown organic compound (which contains only carbon,...
Questions

Mathematics, 19.07.2019 00:00

Mathematics, 19.07.2019 00:00

Social Studies, 19.07.2019 00:00



Chemistry, 19.07.2019 00:00

Advanced Placement (AP), 19.07.2019 00:00

Mathematics, 19.07.2019 00:00

History, 19.07.2019 00:00



Social Studies, 19.07.2019 00:00






Social Studies, 19.07.2019 00:00


Mathematics, 19.07.2019 00:00

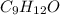 and
and 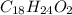
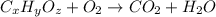
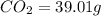
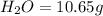
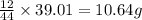 of carbon will be contained.
of carbon will be contained.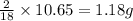 of hydrogen will be contained.
of hydrogen will be contained.