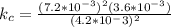A sample of pure NO2NO2 is heated to 335 ∘C∘C at which temperature it partially dissociates according to the equation 2NO2(g)⇌2NO(g)+O2(g)2NO2(g)⇌2NO(g)+ O2(g) At equilibrium the density of the gas mixture is 0.525 g/Lg/L at 0.750 atmatm .Calculate Kc for the reaction

Answers: 1
Another question on Chemistry




Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
You know the right answer?
A sample of pure NO2NO2 is heated to 335 ∘C∘C at which temperature it partially dissociates accordin...
Questions

Mathematics, 28.06.2019 11:10



Mathematics, 28.06.2019 11:10

Social Studies, 28.06.2019 11:10






Mathematics, 28.06.2019 11:10

Mathematics, 28.06.2019 11:10



Mathematics, 28.06.2019 11:10

Mathematics, 28.06.2019 11:10


Biology, 28.06.2019 11:10

Mathematics, 28.06.2019 11:10

Mathematics, 28.06.2019 11:10

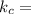 1. 1 × 10⁻²
1. 1 × 10⁻²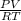
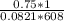
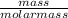
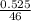
![k_c = \frac{[NO]^2[O_2]}{[NO_2]^2}](/tpl/images/0542/3911/d520a.png)