
The following equation shows the equilibrium in an aqueous solution of ammonia: NH3(aq)+H2O(l)⇌NH4+(aq)+OH−(aq)NH3( aq)+H2O(l)⇌NH4+(aq)+OH−(aq) Which of the following represents a conjugate acid-base pair?a) NH3 and H2O b) NH4+ and OH− c) H2O and OH− d) NH3 and OH−

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
You know the right answer?
The following equation shows the equilibrium in an aqueous solution of ammonia: NH3(aq)+H2O(l)⇌NH4+(...
Questions






Computers and Technology, 12.08.2020 07:01














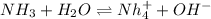
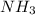 and the conjugate acid of the base is
and the conjugate acid of the base is 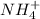 .
.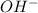 and the conjugate acid of the base is
and the conjugate acid of the base is 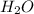 .
.

