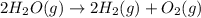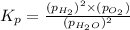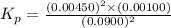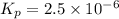Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2


Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
You know the right answer?
The elementary reaction 2H20(g)<--->2H2(g)+O2(g) proceeds at a certain temperature until the p...
Questions

Mathematics, 14.01.2021 19:10


English, 14.01.2021 19:10


Mathematics, 14.01.2021 19:10

Mathematics, 14.01.2021 19:20




Mathematics, 14.01.2021 19:20

Mathematics, 14.01.2021 19:20


Mathematics, 14.01.2021 19:20

Mathematics, 14.01.2021 19:20

English, 14.01.2021 19:20

Mathematics, 14.01.2021 19:20


Physics, 14.01.2021 19:20



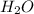 = 0.0900 atm
= 0.0900 atm = 0.00450 atm
= 0.00450 atm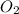 = 0.00100 atm
= 0.00100 atm