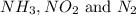Chemistry, 11.03.2020 05:11 kylaprather06
Consider three 1L flask at STP. Flask A contains NH3, flask B contains NO2 gas and flask C contain N2 gas. Which constains the largest number of molecules?

Answers: 1
Another question on Chemistry




Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1
You know the right answer?
Consider three 1L flask at STP. Flask A contains NH3, flask B contains NO2 gas and flask C contain N...
Questions








Mathematics, 27.06.2019 04:30




English, 27.06.2019 04:30





Social Studies, 27.06.2019 04:30

Health, 27.06.2019 04:30

Biology, 27.06.2019 04:30