

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
In the first 11.0 s s of the reaction, 1.6×10−2 mol mol of O2O2 is produced in a reaction vessel wit...
Questions

Mathematics, 13.05.2021 18:50




Computers and Technology, 13.05.2021 18:50






Computers and Technology, 13.05.2021 18:50

Mathematics, 13.05.2021 18:50

Mathematics, 13.05.2021 18:50


Mathematics, 13.05.2021 18:50


Mathematics, 13.05.2021 18:50


History, 13.05.2021 18:50

![\frac{d[O_2]}{dt}](/tpl/images/0542/6081/a384d.png)
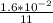 = 1.455×10⁻³ mol/second.
= 1.455×10⁻³ mol/second.


