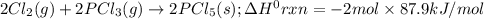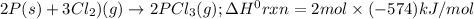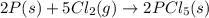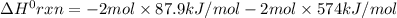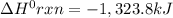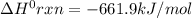Chemistry, 11.03.2020 06:31 cyanezc1313
Consider the following thermochemical equations. PCl5 (s)→PCl3 (g)+Cl2 (g)2P (s)+3Cl2 (g)→2PCl3 (g)ΔH∘rxn=87.9kJmol ΔH∘rxn=−574kJmol Using this data, determine the heat of formation for PCl5.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2
You know the right answer?
Consider the following thermochemical equations. PCl5 (s)→PCl3 (g)+Cl2 (g)2P (s)+3Cl2 (g)→2PCl3 (g)Δ...
Questions


Chemistry, 29.01.2021 09:20



Mathematics, 29.01.2021 09:20



History, 29.01.2021 09:20

Mathematics, 29.01.2021 09:20





Mathematics, 29.01.2021 09:20

Mathematics, 29.01.2021 09:20



Mathematics, 29.01.2021 09:20

Social Studies, 29.01.2021 09:20

Spanish, 29.01.2021 09:20