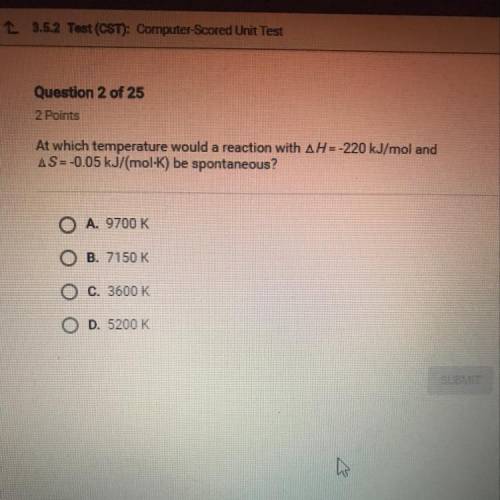
At which temperature would a reaction with AH = -220 kJ/mol and
AS=-0.05 kJ/(mol-K) be spontan...

Chemistry, 11.03.2020 09:51 michaelchavez6959127
At which temperature would a reaction with AH = -220 kJ/mol and
AS=-0.05 kJ/(mol-K) be spontaneous?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 23.06.2019 08:40
The activation energy for this reaction is 75 kj·mol–1. the enzyme catalase (found in blood) lowers the activation energy to 8.0 kj·mol–1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25°c?
Answers: 2
You know the right answer?
Questions

Social Studies, 06.10.2019 20:00

History, 06.10.2019 20:00



History, 06.10.2019 20:00




Biology, 06.10.2019 20:00

Mathematics, 06.10.2019 20:10



Chemistry, 06.10.2019 20:10



Chemistry, 06.10.2019 20:10



Mathematics, 06.10.2019 20:10

World Languages, 06.10.2019 20:10



