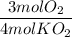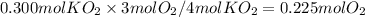The reaction between potassium superoxide, KO₂, and CO₂,
is used as a source of O₂ and...

Chemistry, 11.03.2020 17:25 gadgetady5699
The reaction between potassium superoxide, KO₂, and CO₂,
is used as a source of O₂ and absorber of CO₂ in self-contained breathing equipment used by rescue workers.
How many moles of O₂ are produced when 0.300 mol of KO₂ reacts in this fashion?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 23.06.2019 03:00
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1
You know the right answer?
Questions

Business, 10.06.2020 19:57

Mathematics, 10.06.2020 19:57

Mathematics, 10.06.2020 19:57




Mathematics, 10.06.2020 19:57

Chemistry, 10.06.2020 19:57


Mathematics, 10.06.2020 19:57

Mathematics, 10.06.2020 19:57

English, 10.06.2020 19:57





Mathematics, 10.06.2020 19:57

Computers and Technology, 10.06.2020 19:57