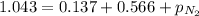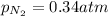Chemistry, 11.03.2020 18:22 makrosebud7821
A mixture of He, NE, and N2 gases has a pressure of 1.043 atm. If the pressures of He and Ne are 0.137 atm and 0.566 atm, respectively, what is the partial pressure of N2 in the mixture?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1

Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
A mixture of He, NE, and N2 gases has a pressure of 1.043 atm. If the pressures of He and Ne are 0.1...
Questions





Mathematics, 23.07.2019 08:30

Advanced Placement (AP), 23.07.2019 08:30


Mathematics, 23.07.2019 08:30



World Languages, 23.07.2019 08:30

Mathematics, 23.07.2019 08:30


World Languages, 23.07.2019 08:30


History, 23.07.2019 08:30

Chemistry, 23.07.2019 08:30



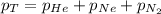
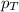 = total pressure of gas = 1.043 atm
= total pressure of gas = 1.043 atm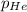 = partial pressure of helium gas = 0.137 atm
= partial pressure of helium gas = 0.137 atm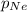 = partial pressure of neon gas = 0.566 atm
= partial pressure of neon gas = 0.566 atm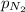 = partial pressure of nitrogen gas = ?
= partial pressure of nitrogen gas = ?