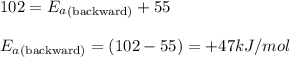Chemistry, 11.03.2020 19:05 lizbethh62
The decomposition of dinitrogen pentaoxide has an activation energy of 102 kJ/mol and ΔH°rxn = + 55 kJ/mol. What is the activation energy for the reverse reaction?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2


Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
You know the right answer?
The decomposition of dinitrogen pentaoxide has an activation energy of 102 kJ/mol and ΔH°rxn = + 55...
Questions




Biology, 21.11.2019 20:31


Biology, 21.11.2019 20:31


Law, 21.11.2019 20:31

Mathematics, 21.11.2019 20:31







History, 21.11.2019 20:31




Mathematics, 21.11.2019 20:31

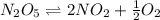
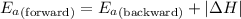
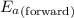 = Activation energy of the forward reaction = 102 kJ/mol
= Activation energy of the forward reaction = 102 kJ/mol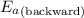 = Activation energy of the backward reaction = ?
= Activation energy of the backward reaction = ?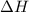 = Enthalpy of the reaction = +55 kJ/mol
= Enthalpy of the reaction = +55 kJ/mol