
Chemistry, 11.03.2020 21:14 kolbehoneyman
An old antacid commercial claimed that each tablet of their product could neutralize 47 times its mass in stomach acid. The active ingredient in the antacid tablet, NaAl ( OH ) 2 CO 3 , NaAl(OH)2CO3, reacts with the HCl HCl in stomach acid according to the balanced reaction here. NaAl ( OH ) 2 CO 3 + 4 HCl ⟶ NaCl + AlCl 3 + 3 H 2 O + CO 2 NaAl(OH)2CO3+4HCl⟶NaCl+AlCl3+3H2O+C O2 How many moles of HCl HCl can a 1.24 1.24 g antacid tablet neutralize if the tablet contains 0.296 0.296 g of the active ingredient?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1
You know the right answer?
An old antacid commercial claimed that each tablet of their product could neutralize 47 times its ma...
Questions

Biology, 29.08.2019 17:30


Mathematics, 29.08.2019 17:30

Mathematics, 29.08.2019 17:30

History, 29.08.2019 17:30




Mathematics, 29.08.2019 17:30


Biology, 29.08.2019 17:30


English, 29.08.2019 17:30


Chemistry, 29.08.2019 17:30

Physics, 29.08.2019 17:30




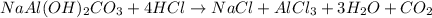
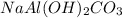
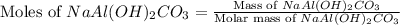
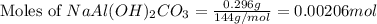
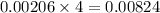 moles of HCl
moles of HCl


