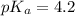Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3


Chemistry, 23.06.2019 11:00
The lab procedure involves several factors, listed below some were variable and some were constant. label each factor below v for variable ot c for constant
Answers: 1

You know the right answer?
An unknown acid is titrated with a strong base. At the half-equivalence point (i. e., at the volume...
Questions




SAT, 30.10.2019 06:31














History, 30.10.2019 06:31


French, 30.10.2019 06:31

![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0543/2312/e961a.png)