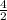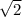Chemistry, 11.03.2020 21:32 jaydqueen3155
The Kc for the following reaction at 100 °C is 2.0. If the equilibrium mixture contains 2.0 M NO and 1.0 M Br2, what is the molar concentration of NOBr?
2NOBr (g) ⇌ 2NO(g) + Br2 (g)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

Chemistry, 23.06.2019 10:40
Question 17 hydrogen is manufactured on an industrial scale by this sequence of reactions: +ch4gh2og ⇌ +cog3h2g k1 +cogh2og ⇌ +co2gh2g k2 the net reaction is: +ch4g2h2og ⇌ +co2g4h2g k write an equation that gives the overall equilibrium constant k in terms of the equilibrium constants k1 and k2. if you need to include any physical constants, be sure you use their standard symbols, which you'll find in the aleks calculator.
Answers: 2
You know the right answer?
The Kc for the following reaction at 100 °C is 2.0. If the equilibrium mixture contains 2.0 M NO and...
Questions



Chemistry, 05.05.2020 23:29

English, 05.05.2020 23:29

History, 05.05.2020 23:29


Mathematics, 05.05.2020 23:29

Geography, 05.05.2020 23:29




Mathematics, 05.05.2020 23:29

Mathematics, 05.05.2020 23:29


Mathematics, 05.05.2020 23:29

Computers and Technology, 05.05.2020 23:29


Spanish, 05.05.2020 23:29


![\frac{[NO]^{2}[Br_{2}]}{[NOBr]^{2}}](/tpl/images/0543/2703/7b851.png)
![\frac{[NO]^{2}[Br_{2}]}{Kc}](/tpl/images/0543/2703/d446e.png)
![\frac{[2]^{2}[1]}{2}](/tpl/images/0543/2703/09250.png)