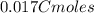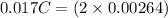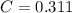Chemistry, 11.03.2020 22:15 hiiliohi1018
An aqueous solution of perchloric acid is standardized by titration with a 0.191 M solution of barium hydroxide. If 13.8 mL of base are required to neutralize 17.0 mL of the acid, what is the molarity of the perchloric acid solution?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3
You know the right answer?
An aqueous solution of perchloric acid is standardized by titration with a 0.191 M solution of bariu...
Questions



Chemistry, 04.06.2020 19:06

English, 04.06.2020 19:06


English, 04.06.2020 19:06






Mathematics, 04.06.2020 19:06




Chemistry, 04.06.2020 19:06

Mathematics, 04.06.2020 19:06


Mathematics, 04.06.2020 19:06

Mathematics, 04.06.2020 19:06

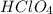 solution is 0.311 M
solution is 0.311 M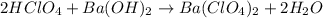
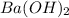 neutralizes 2 moles of
neutralizes 2 moles of 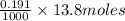 =
= 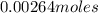
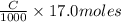 =
=