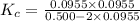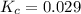Chemistry, 11.03.2020 22:49 webbjalia04
At high temperature, 2.00 mol of HBr was placed in a 4.00 L container where it decomposed in the reaction: 2HBr(g) H2(g) Br2(g) At equilibrium the concentration of Br2 was measured to be 0.0955 M. What is Kc for this reaction at this temperature

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3


Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1
You know the right answer?
At high temperature, 2.00 mol of HBr was placed in a 4.00 L container where it decomposed in the rea...
Questions

Mathematics, 13.05.2021 17:20

English, 13.05.2021 17:20


History, 13.05.2021 17:20

Mathematics, 13.05.2021 17:20


Mathematics, 13.05.2021 17:20

Mathematics, 13.05.2021 17:20

French, 13.05.2021 17:20

Mathematics, 13.05.2021 17:20


History, 13.05.2021 17:20


Mathematics, 13.05.2021 17:20

Mathematics, 13.05.2021 17:20

English, 13.05.2021 17:20


Mathematics, 13.05.2021 17:20


Mathematics, 13.05.2021 17:20

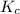 for this reaction at this temperature is 0.029
for this reaction at this temperature is 0.029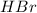 = 2.00 mole
= 2.00 mole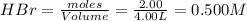
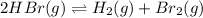
![K_c=\frac{[H_2\times [Br_2]}{[HBr]^2}](/tpl/images/0543/5528/3a3d4.png)
![[Br_2]](/tpl/images/0543/5528/f23ed.png) = x = 0.0955 M
= x = 0.0955 M