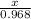Chemistry, 11.03.2020 22:59 cthompson1107
According to the following reaction, how many grams of nitrogen monoxide will be formed upon the complete reaction of 31.0 grams of oxygen gas with excess ammonia? ammonia (g) + oxygen (g) nitrogen monoxide (g) + water (g)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:30
10. according to the law of conservation of mass, how does the mass of the products in a chemical reaction compare to the mass of the reactants?
Answers: 3

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
According to the following reaction, how many grams of nitrogen monoxide will be formed upon the com...
Questions

Social Studies, 12.12.2019 03:31

World Languages, 12.12.2019 03:31


Biology, 12.12.2019 03:31


Business, 12.12.2019 03:31



Mathematics, 12.12.2019 03:31

Mathematics, 12.12.2019 03:31

History, 12.12.2019 03:31

History, 12.12.2019 03:31


English, 12.12.2019 03:31


History, 12.12.2019 03:31




History, 12.12.2019 03:31

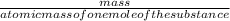
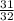
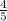 =
=