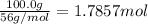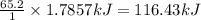Chemistry, 11.03.2020 22:59 isabellacampos4586
Calcium oxide reacts with water to produce calcium hydroxide and 65.2 kJ of heat in the following reaction. CaO (s) + H2O (l) → Ca(OH)2 (s) ΔH = - 65.2 kJ How much heat is released when 100.0 g of calcium oxide reacts with excess water?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2

Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1
You know the right answer?
Calcium oxide reacts with water to produce calcium hydroxide and 65.2 kJ of heat in the following re...
Questions




Mathematics, 10.05.2021 23:10

Business, 10.05.2021 23:10

Mathematics, 10.05.2021 23:10

Chemistry, 10.05.2021 23:10


Social Studies, 10.05.2021 23:10

Mathematics, 10.05.2021 23:10

Mathematics, 10.05.2021 23:10


Social Studies, 10.05.2021 23:10

Mathematics, 10.05.2021 23:10






Mathematics, 10.05.2021 23:10

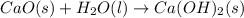 , ΔH = -65.2 kJ
, ΔH = -65.2 kJ