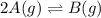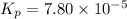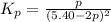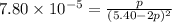Consider the reaction.
2A(g)−⇀↽−B(g) Kp=7.80×10−5
at 500 K If a sample of A(g) at 5.40 a...


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

Chemistry, 23.06.2019 04:31
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1
You know the right answer?
Questions

Health, 03.02.2020 07:51

Mathematics, 03.02.2020 07:51






Mathematics, 03.02.2020 07:51

Geography, 03.02.2020 07:51

History, 03.02.2020 07:51

History, 03.02.2020 07:51

Mathematics, 03.02.2020 07:51

English, 03.02.2020 07:51


Chemistry, 03.02.2020 07:51

Mathematics, 03.02.2020 07:52

History, 03.02.2020 07:52



Mathematics, 03.02.2020 07:52