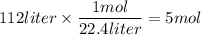Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Figure 10-1 study figure 10-1. the strong nuclear force felt by a single proton in a large nucleus
Answers: 3

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

You know the right answer?
Use the chemical equation to help calculate the mass in grams of zinc needed to produce 112 liters o...
Questions



Mathematics, 18.05.2021 02:30

Mathematics, 18.05.2021 02:30


Mathematics, 18.05.2021 02:40




Social Studies, 18.05.2021 02:40

Physics, 18.05.2021 02:40


History, 18.05.2021 02:40

Physics, 18.05.2021 02:40


Mathematics, 18.05.2021 02:40

Mathematics, 18.05.2021 02:40


History, 18.05.2021 02:40